Steel as an Iron Alloy
Steel is a mixture of iron with other elements, the so-called iron companions. These elements are also called alloying elements and the resulting mixture is called alloy. All iron materials with a carbon content of less than 2 percent that can be forged without subsequent treatment are called steel. Additionally we differentiate between alloyed and unalloyed steel depending on the proportion of other alloying elements.
The alloying elements can have a very significant influence on the properties of the finished product. For example, stainless steel is an iron alloybased on about 20 percent chromium. Acid-resistant steel is based on the addition of silicon, and for manganese steel the iron is alloyed with 0.8 to 14 percent manganese.
An Important Tool: The Iron-Carbon-Diagram
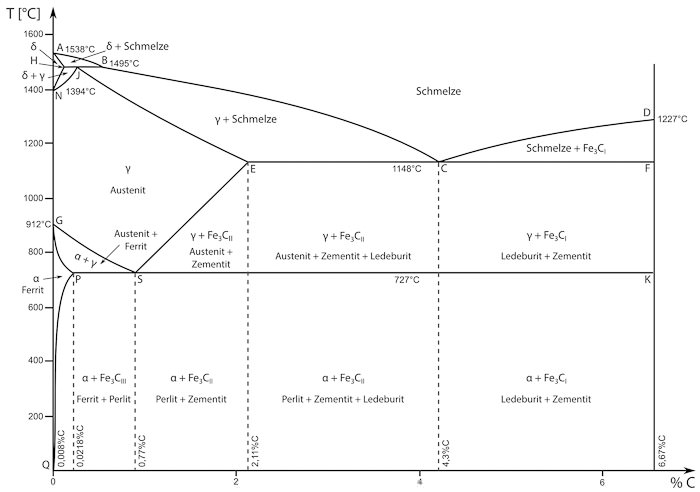
The iron-carbon-diagram contains information about the microstructure of iron in relation to temperature and carbon content.
These areas of the diagram are especially noteworthy:
- At room temperature iron exists as ferrite (alpha-iron).
- Perlite contains a low amount of carbon with lamellar distribution.
- Austenite (gamma-iron) has a different atomic lattice and usually exists at temperatures above 727°C. Austenite can dissolve much more carbon than ferrite or perlite.
- Delta-ferrite only exists at very high temperatures and low amounts of carbon. It does not have relevant technical applications.
- Cementite is another name for iron carbide(Fe3C).It deposits at the grain boundaries when the solubility limit for carbon is reached.

The Microstructure Influences Material Properties
Knowledge about the microstructure of iron alloys is important because it enables us to predict some properties of a specific steel grade. Pure iron for example has a body-centred cubic lattice and therefore is very soft and magnetic. However, it is rather difficult to produce due to the low amount of contaminants.
In comparison austenite has a face-centred cubic lattice and can dissolve much more carbon. It is not magnetic but, similar to ferrite, very soft and easily malleable. Usually austenite only exists at temperatures above 727°C but some alloying elements such as nickel, cobalt and manganese allow it to exist at room temperature.
Cementite is another important metallographic constituent. It contains 6.67 percent carbon and is extremely hard and brittle. Therefore, it is very wear-resistant; it prevents mechanical deformation, however.
Perlite is a eutectoid microstructure created at 0.8 percent carbon. It has a lamellar structure with interchanging areas of ferrite and cementite.
The eutectic structure of iron and carbon is called ledeburite. It contains 4.3 percent carbon and Ledeburit enables a clear distinction between steel and cast iron. Pure ledeburite is badly malleable but there are some forgeable grades.
More Variation Through Heat Treatment
The microstructures described are formed when the material is cooled infinitely slowly. Heat treatment influences the temperature gradation and can create different microstructures. The heat treatment process usually involves three steps: controlled heating, holding at a specified temperature and controlled cooling.
Martensite for example is created using a heat treatment process with a very fast cooling phase. Martensite is very hard and brittle. Sometimes heat-treatment is also applied to change factors other than the microstructure.